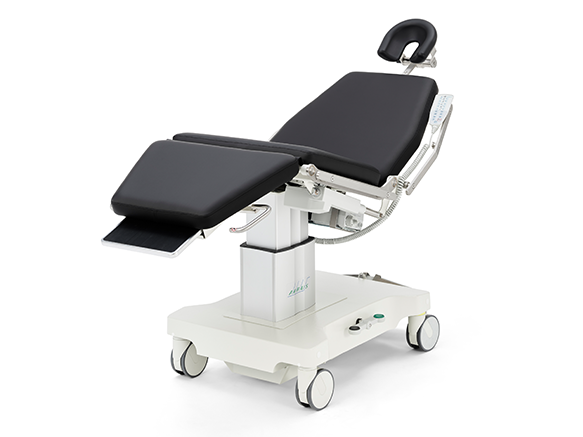
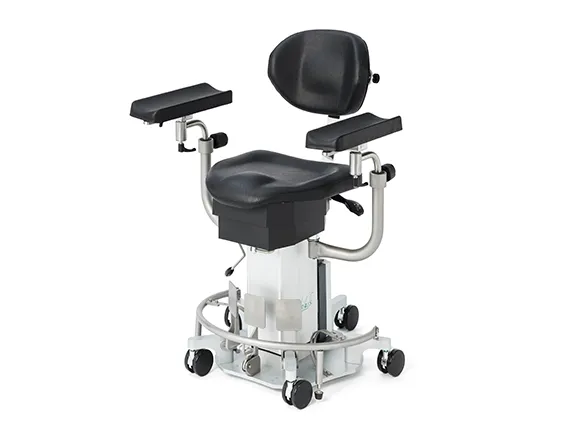
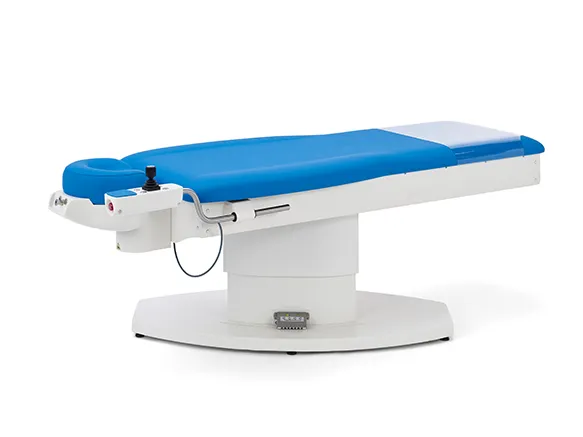
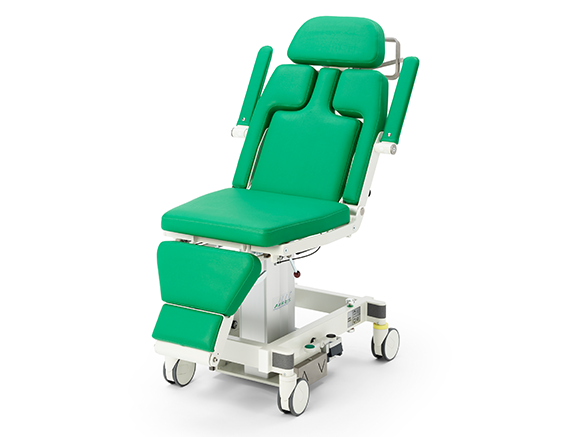
PATIENT POSITIONING SYSTEMS
MADE IN GERMANY

We supply sustainable patient positioning solutions for outpatient surgical techniques in various medical specialties. Our production and administration site is located in Elmshorn near Hamburg in Germany.
"With a great deal of commitment, many years of experience and process responsibility across the entire production chain, we manufacture high-quality products with a special price-performance ratio. AKRUS stands for great employees with a keen sense for customer wishes and solutions. They successfully meet the challenges of a medium-sized production company every day. I thank them very much for that."
Rainer Höpfl, Managing Director AKRUS GmbH & Co. KG
In addition to our own products, which are distributed via a worldwide network of specialist dealers, we also manufacture on behalf of well-known global companies in the medical technology industry. Our services include design & development, production & assembly, product registration, shipping organization and after-sales service.
At AKRUS, we support surgeons and industry partners around the world with flexible, mobile and independent patient positioning solutions for outpatient procedures.
We are proud to make our contribution to ergonomic workflows and precise surgical results for patients.
Patient positioning systems for outpatient medical applications
We support international customers with design and development proposals. AKRUS plans, integrates electronic interfaces and provides prototypes. We also take care of product registration or support customers in their registration processes.
Design, development and sales with expertise and experience
Many years of experience and expertise are reflected in all our products. From brainstorming to dispatch processing. AKRUS stands for functional design, ergonomic workflows, reliable use and cost-effectiveness in purchase and maintenance.
High vertical range of manufacture and short delivery routes
At AKRUS, we take advantage of the high level of vertical integration and keep the process chain in our own hands. This allows us to react quickly and flexibly to market changes and closely monitor product quality. In terms of sustainability, we pay attention to short delivery routes from our suppliers.
Assembly and product testing
Operating chairs and tables are assembled in specialized assembly departments. Our complex patient positioning systems are expertly assembled and tested by hand. After assembly, each product receives its own test certificate and is securely packaged.
Shipping at home and abroad
Our location in Elmshorn, in the immediate vicinity of Hamburg, is a logistical advantage for our customers. We have cooperations with national and international carriers by air, land and sea. With our detailed knowledge in the national and international area, we can solve any shipping request.
Mobile
AKRUS operating chairs and tables are mobile and mains-independent to ensure the greatest possible flexibility. Patients can be prepared for surgery on the chairs, wheeled into the operating theater, operated on and then discharged in a controlled manner. The battery operation of the position adjustment motors ensures complete independence and mobility in the operating theater environment. Large, smooth-running castors ensure easy maneuvering of the operating chair, even with patients sitting or lying down.
Ergonomic.
In the daily routine of outpatient surgery, it is necessary to be able to work in a relaxed and fatigue-free manner. The ergonomically sensible adjustment of the operating field is carried out by battery-powered electric motors of the various segments and thus supports professional work.
Reliable.
We are aware of the need to be able to rely on the full function of an operating chair or operating table - for many years to come. The reliable, durable and resilient design of AKRUS products is the basis for many years of intended use. In addition, only electronic components from renowned manufacturers are used.
Our customers and users appreciate the economical purchase and maintenance of our products.
Management System Certificate
AKRUS is certified in accordance with ISO 13485:2016 and the products we sell are approved as Class 1 medical devices. This means that we comply with the regulations of the European MDR (Medical Device Regulation). We offer CE certified products. The advantages for our customers and users are, for example, guaranteed batch traceability, notification of product and parts deliveries, a stable and consistent manufacturing process and risk minimization through process orientation. For our specialist trade customers, our certification also means that they can dispense with the supplier audit.
Worldwide distribution via a select dealer network.
AKRUS distributes its products exclusively via authorized specialist dealers.
- Competent contacts with regional knowledge
- Fast response speed for local users
- Feedback on market needs
- Cost advantages by not having your own distribution network
- Product training courses for specialist dealers
- Guaranteed technical service