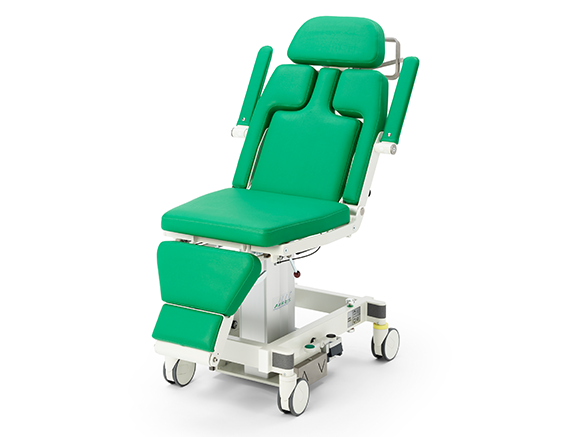
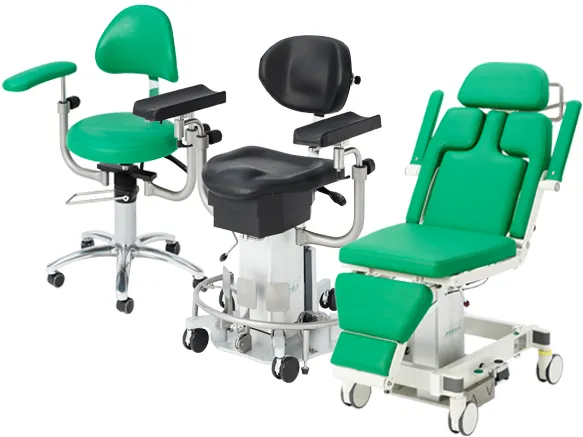
PRODUCT HIGHLIGHTS

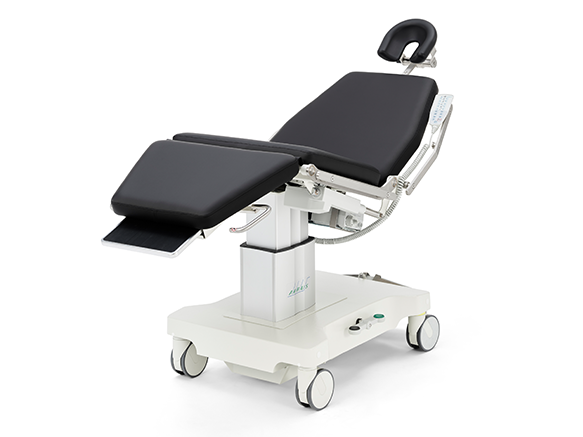
ak5010 MBS
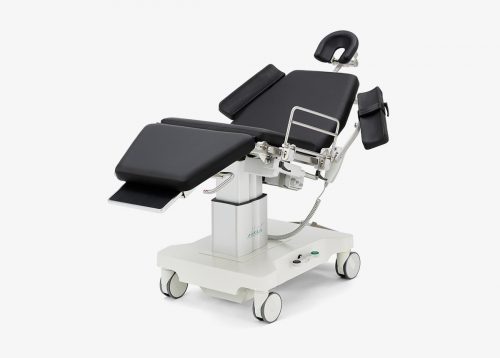
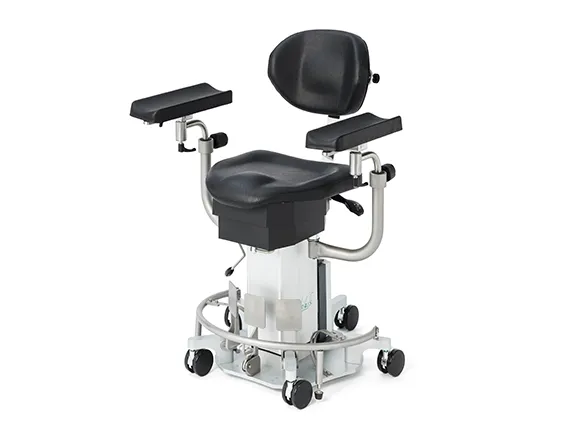
sc5010 ES
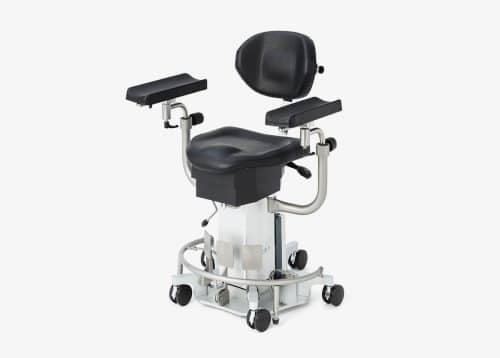
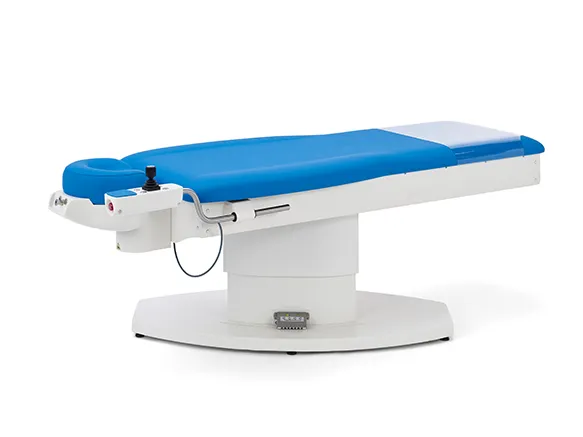
ak480
WE OFFER MOBILE SOLUTIONS
Our mission is to support surgeons and industry partners around the world with patient positioning systems that enable precise surgical outcomes with an ergonomic workflow during outpatient procedures.
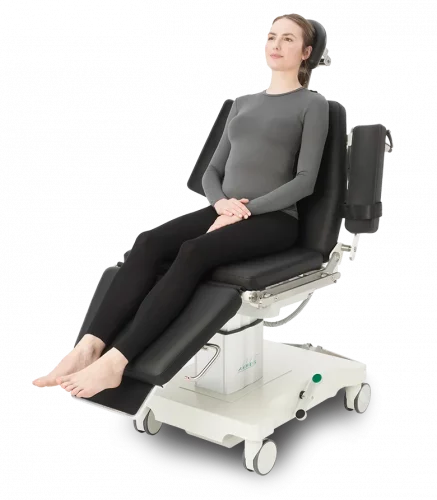
- Mobile operating chairs & tables for outpatient procedures
- Flexible and ergonomic workflows
- Accessories for functional expansion
The ak 5010 MBS is a technically and ergonomically specially developed chair for positioning and transporting patients.
Typical areas of application are ENT, MKG. Plastic, reconstructive and aesthetic surgery (head area) as well as nerve stimulation and blood donation.
MOBILE.
ERGONOMIC.
RELIABLE.

AKRUS GmbH & Co KG
DISCOVER OUR PRODUCTS
Management System Certificate
AKRUS is certified in accordance with ISO 13485:2016 and the products we sell are approved as Class 1 medical devices. This means that we comply with the regulations of the European MDR (Medical Device Regulation). We offer CE certified products. The advantages for our customers and users are, for example, guaranteed batch traceability, notification of product and parts deliveries, a stable and consistent manufacturing process and risk minimization through process orientation. For our specialist trade customers, our certification also means that they can dispense with the supplier audit.